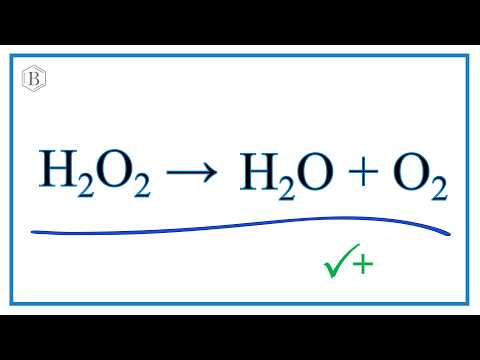
In Situ OH Generation from O2− and H2O2 Plays a Critical Role in Plasma-Induced Cell Death | PLOS ONE
Why is the answer B? Can someone explain this to me and why other options are incorrect. I assumed that H2O2 will decompose rapidly to form H20 and O2 with MnO2 as
How will you show that H2O2 acts as both oxidising and reducing agent? What is meant by '30 volume' of H2O2? - Quora

Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect
What is the balanced half-reaction equation for H2O2 (aq) acting as a reducing agent in an acidic aqueous solution? - Quora

Direct Synthesis of H2O2 from H2 and O2 on Pd Catalysts: Current Understanding, Outstanding Questions, and Research Needs | ACS Catalysis
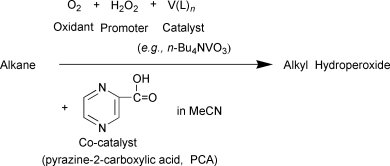
Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 12.1 Main features, kinetics and mechanism of alkane hydroperoxidation - Journal of the Chemical Society, Perkin Transactions 2 (RSC Publishing)

Machine learning–based inverse design for electrochemically controlled microscopic gradients of O2 and H2O2 | PNAS
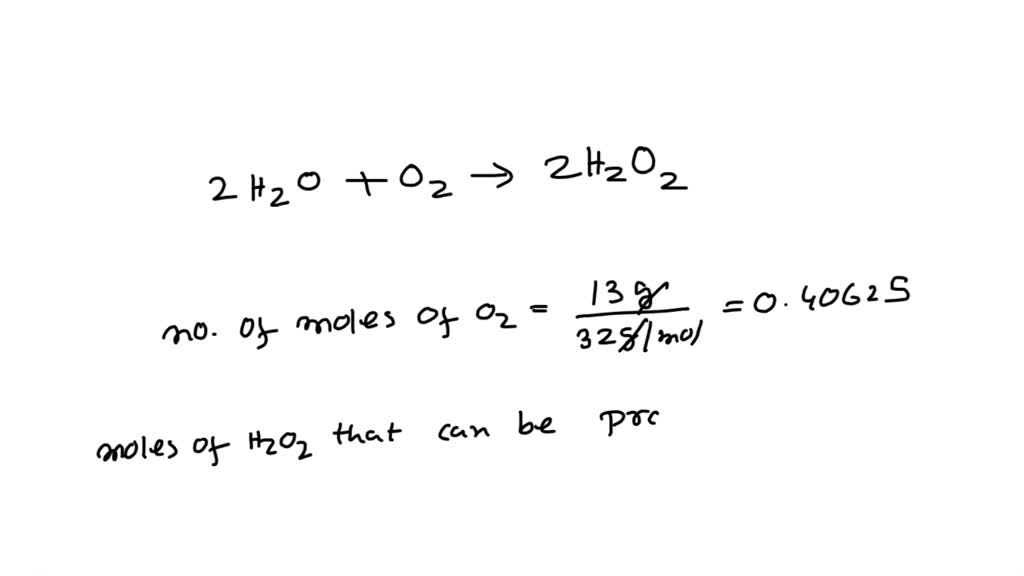
SOLVED: How many moles of H2O2 are produced in the production of hydrogen peroxide when 13 grams of oxygen are used?2 H2O+O2→2 H2O2 81 g H2O2 6.5 mol H2O2 0.81 mol H2O2 13 mol H2O2
I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community