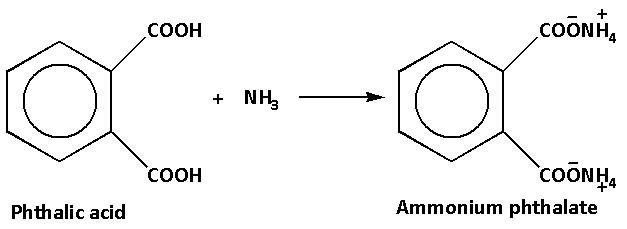
text{Phthalic acid}} + {\\text{N}}{{\\text{H}}_3} \\to {\\text{D}}\\xrightarrow{{{\\Delta }}}{\\text{E}}$
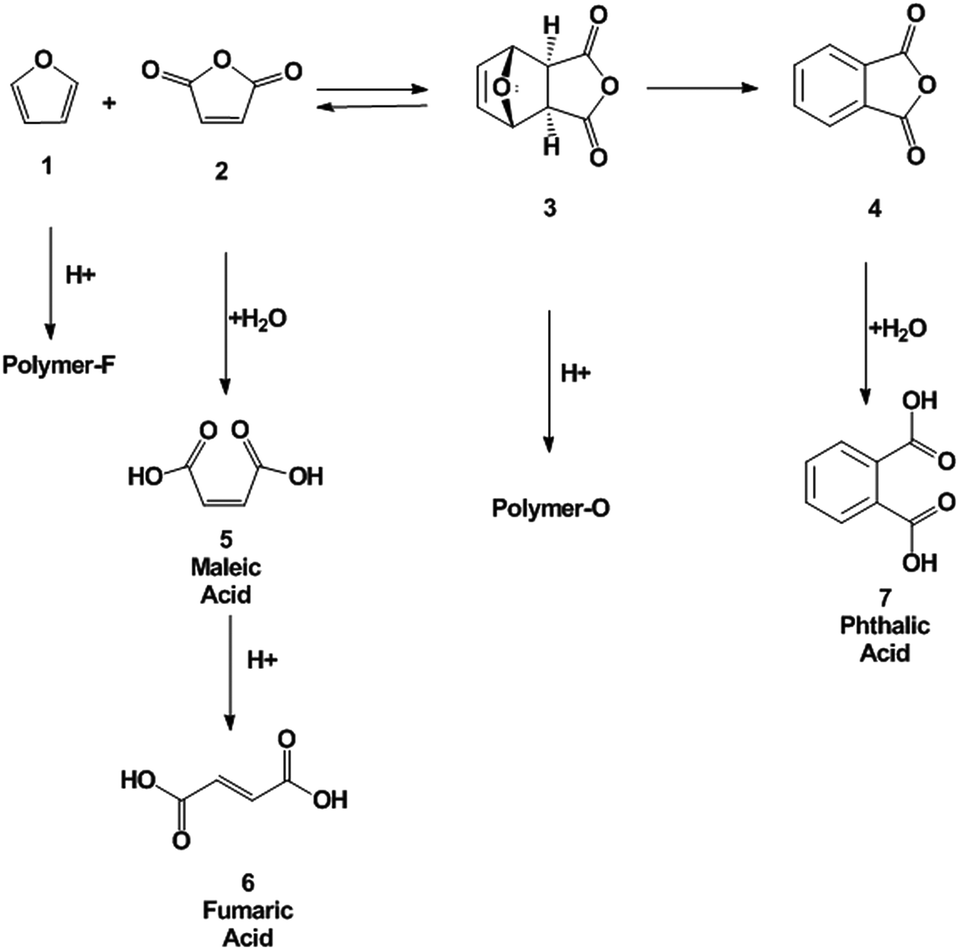
Renewable production of phthalic anhydride from biomass-derived furan and maleic anhydride - Green Chemistry (RSC Publishing) DOI:10.1039/C3GC41655K

Time evolution of product (3) and intermediate (12) selectivity. The... | Download Scientific Diagram
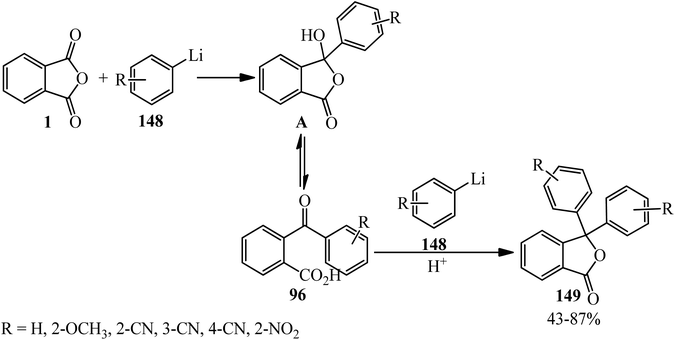
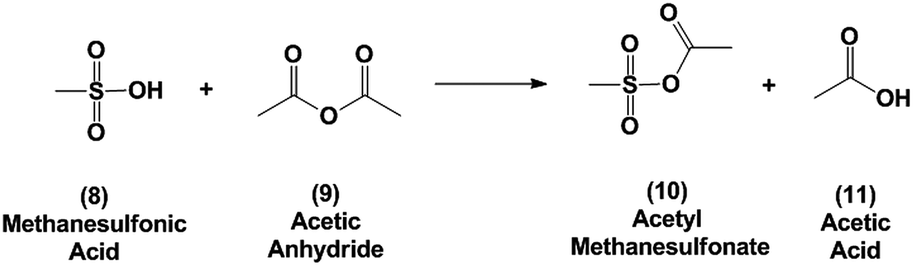
Phthalic anhydride (PA): a valuable substrate in organic transformations - RSC Advances (RSC Publishing) DOI:10.1039/D3RA03378C

Plasticizers: Synthesis of phthalate esters via FeCl3-catalyzed nucleophilic addition of alcohols to phthalic anhydride - ScienceDirect
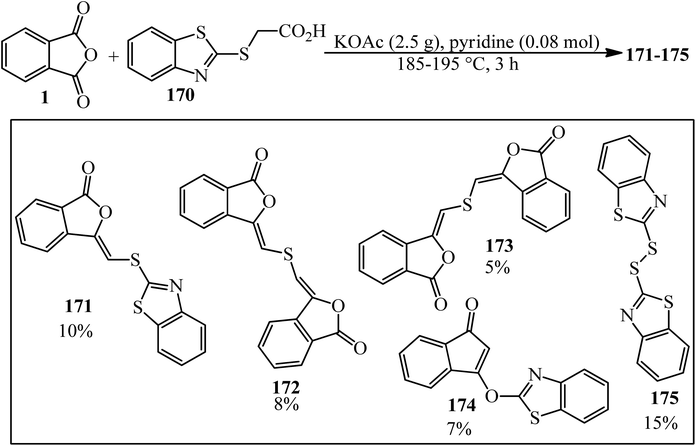
Phthalic anhydride (PA): a valuable substrate in organic transformations - RSC Advances (RSC Publishing) DOI:10.1039/D3RA03378C
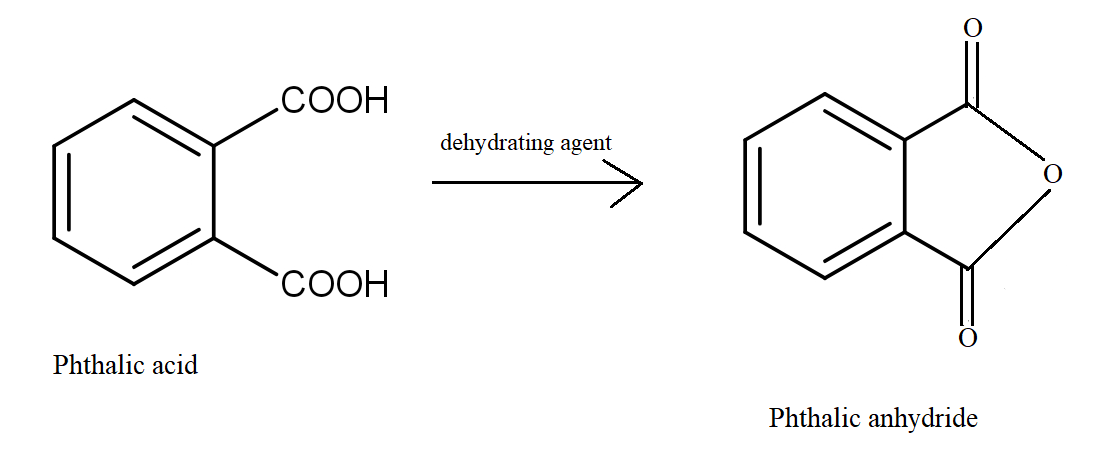
Which dicarboxylic acid in presence of a dehydrating agent is least reactive to give an anhydride?A.\n \n \n \n \n B.\n \n \n \n \n C.\n \n \n \n \n D.\n \n \

Propose an arrow-pushing mechanism for the reaction between phthalic anhydride, nitric acid, and sulfuric acid to produce 3-nitrophthalic acid. | Homework.Study.com
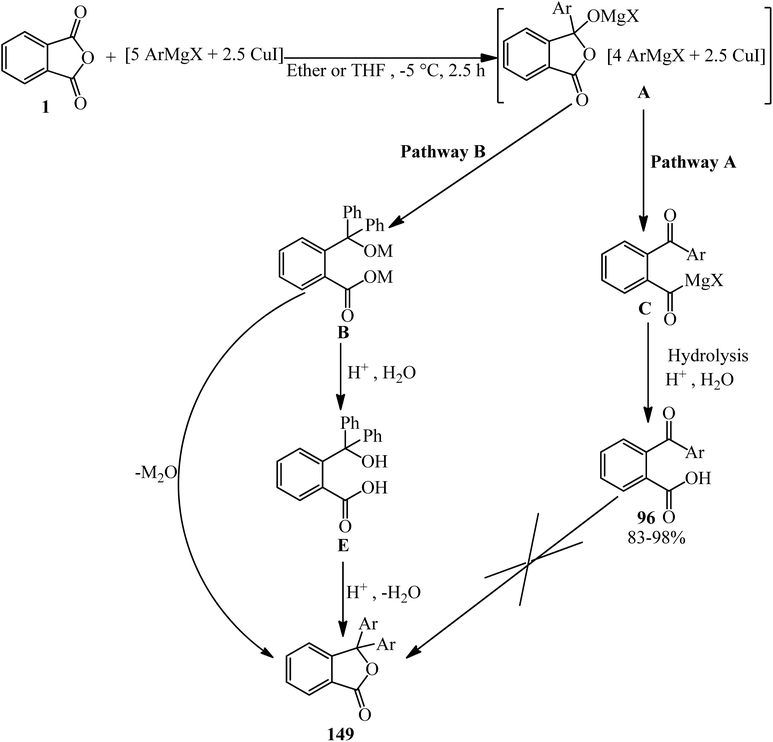
Phthalic anhydride (PA): a valuable substrate in organic transformations - RSC Advances (RSC Publishing) DOI:10.1039/D3RA03378C
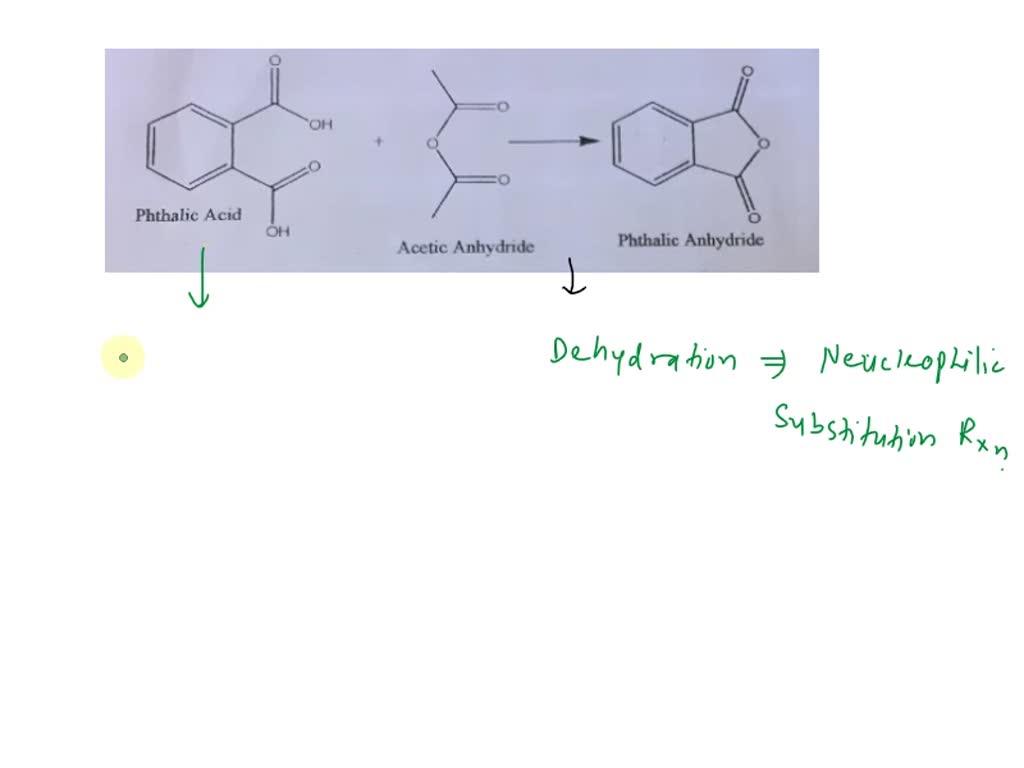
SOLVED: What type of reaction would be used to form phthalic anhydride from phthalic acid and acetic anhydride? And what is the nucleophile, electrophile, substrate, activating or deactivating species?
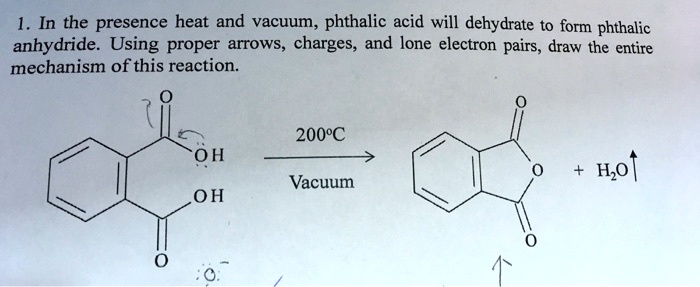
SOLVED: In the presence heat and vacuum phthalic acid will dehydrate to form phthalic anhydride. Using proper arrows, charges, and lone electron pairs, draw the entire mechanism of this reaction 200"C 0H

organic chemistry - Why is there no such substance as p-phthalic anhydride - Chemistry Stack Exchange