Environmentally Friendly, Co-catalyst-Free Chemical Fixation of CO2 at Mild Conditions Using Dual-Walled Nitrogen-Rich Three-Dimensional Porous Metal–Organic Frameworks | Inorganic Chemistry

Supercritical carbon dioxide technology in synthesis, modification, and recycling of battery materials - Han - Carbon Neutralization - Wiley Online Library
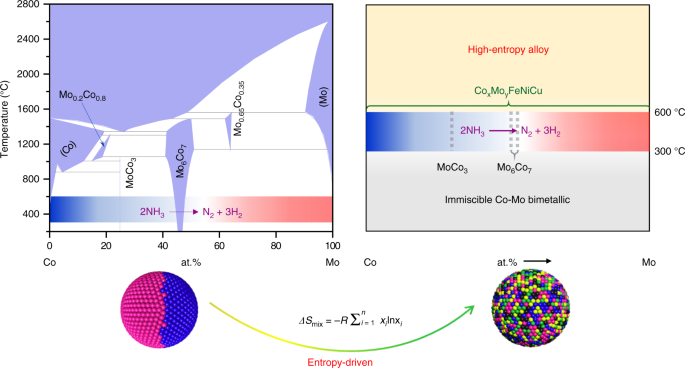
Highly efficient decomposition of ammonia using high-entropy alloy catalysts | Nature Communications

Calculate the entropy change when 2.8g of N2 gas expands isothermally and reversibly from an initial volume of 1L to final volume of 10L at 27^∘ C

Metal–CO2 Electrochemistry: From CO2 Recycling to Energy Storage - Wang - 2021 - Advanced Energy Materials - Wiley Online Library
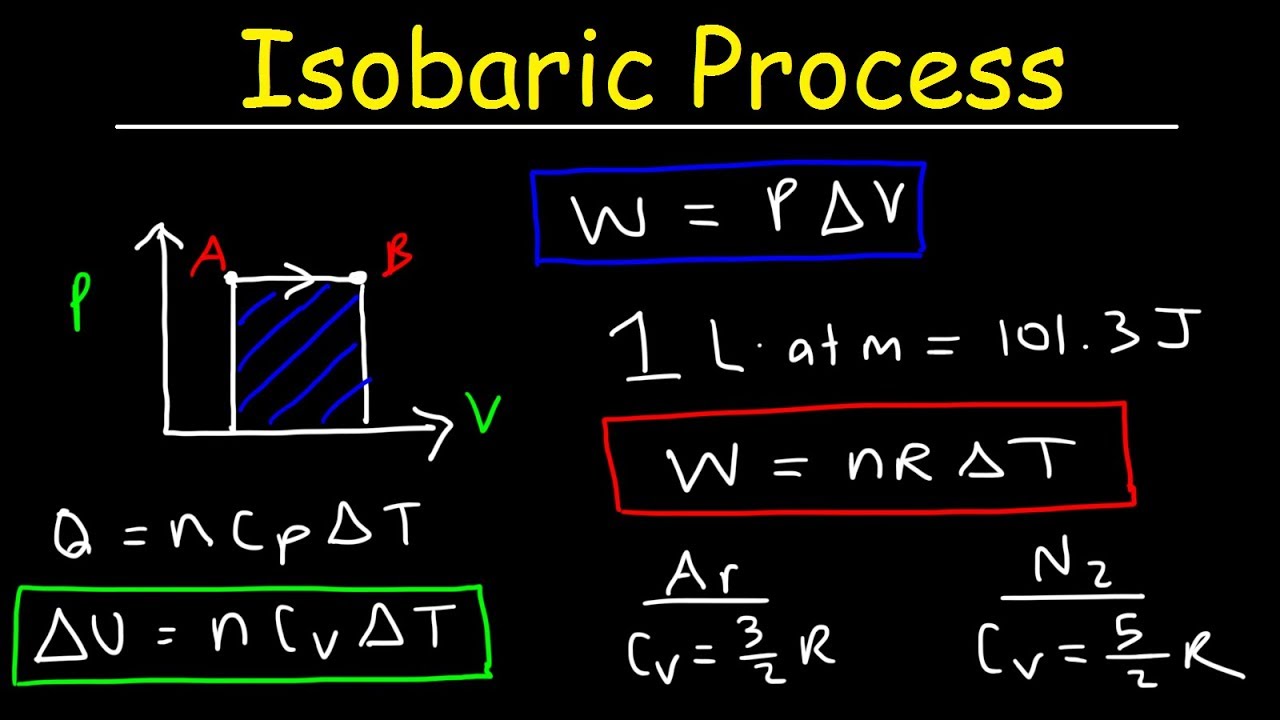
Isobaric Process Thermodynamics - Work & Heat Energy, Molar Heat Capacity, & Internal Energy - YouTube
How to calculate the number of molecules of oxygen gas that occupies a volume of 224 ml at 273k and 3 atm - Quora
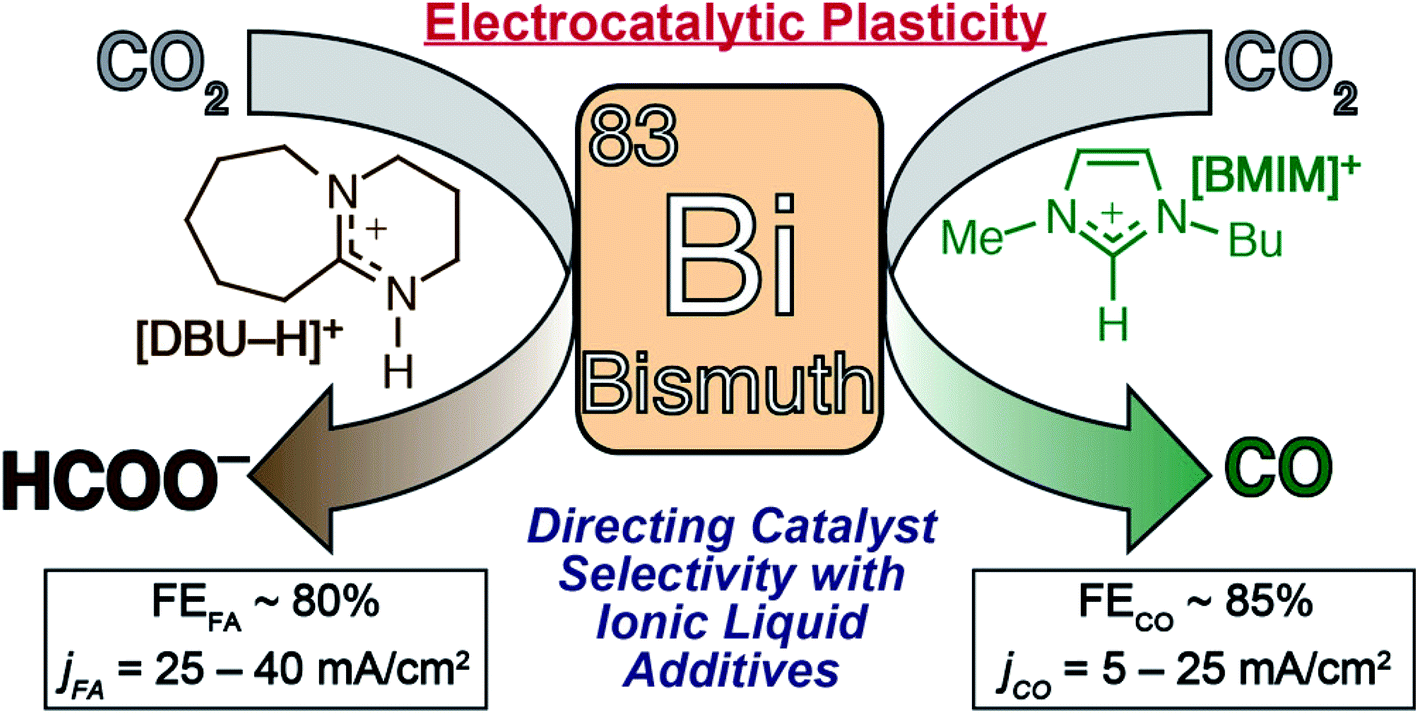
Electrochemical reduction of carbon dioxide (CO 2 ): bismuth-based electrocatalysts - Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/D1TA01516H

22 g of CO2 at 27^0 C is mixed in a closed container with 16 g of O2 at 37^0 C. It both gases are considered as ideal kinetic theory gases, then
DOT US Department of Transportation PHMSA Pipeline and Hazardous Materials Safety Administration OPS Office of Pipeline Safety

Calculate the volume occupied by 8.8 g of CO2 at 31.1^0C and 1 bar pressure. R = 0.083 bar litre K^-1 mole^-1 .

Supercritical carbon dioxide technology in synthesis, modification, and recycling of battery materials - Han - Carbon Neutralization - Wiley Online Library