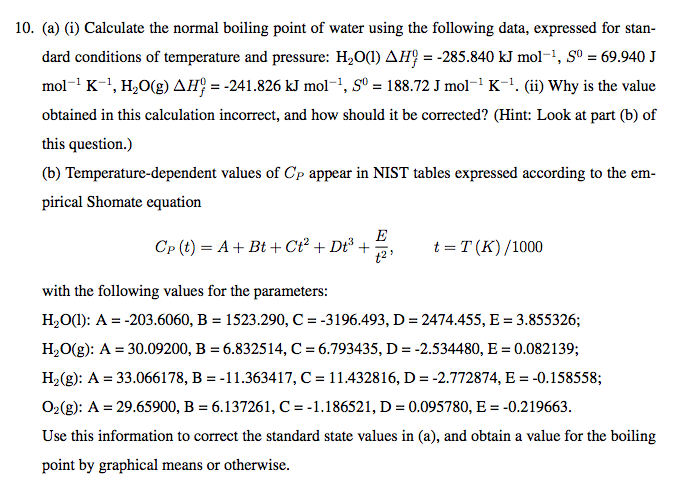
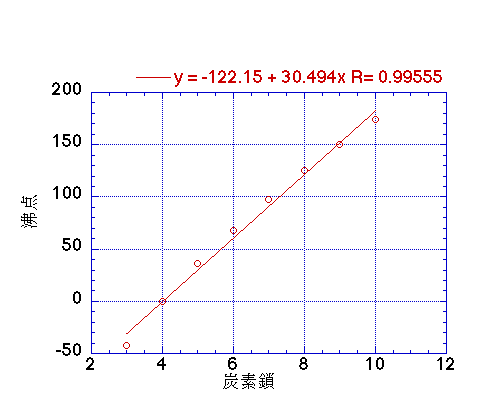
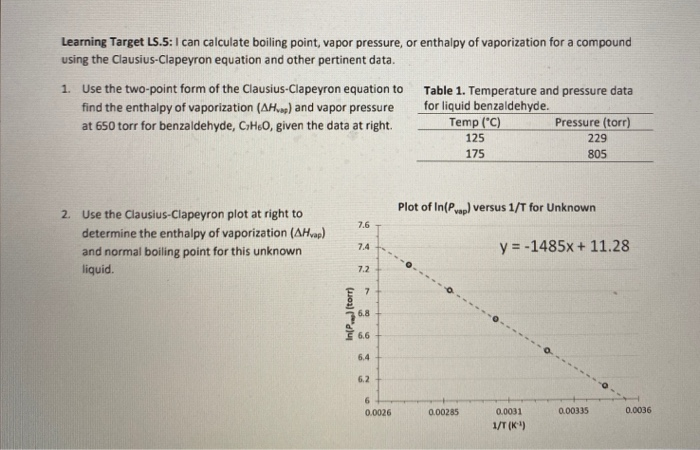
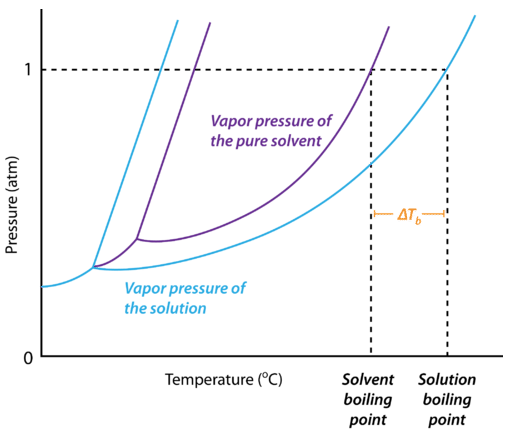
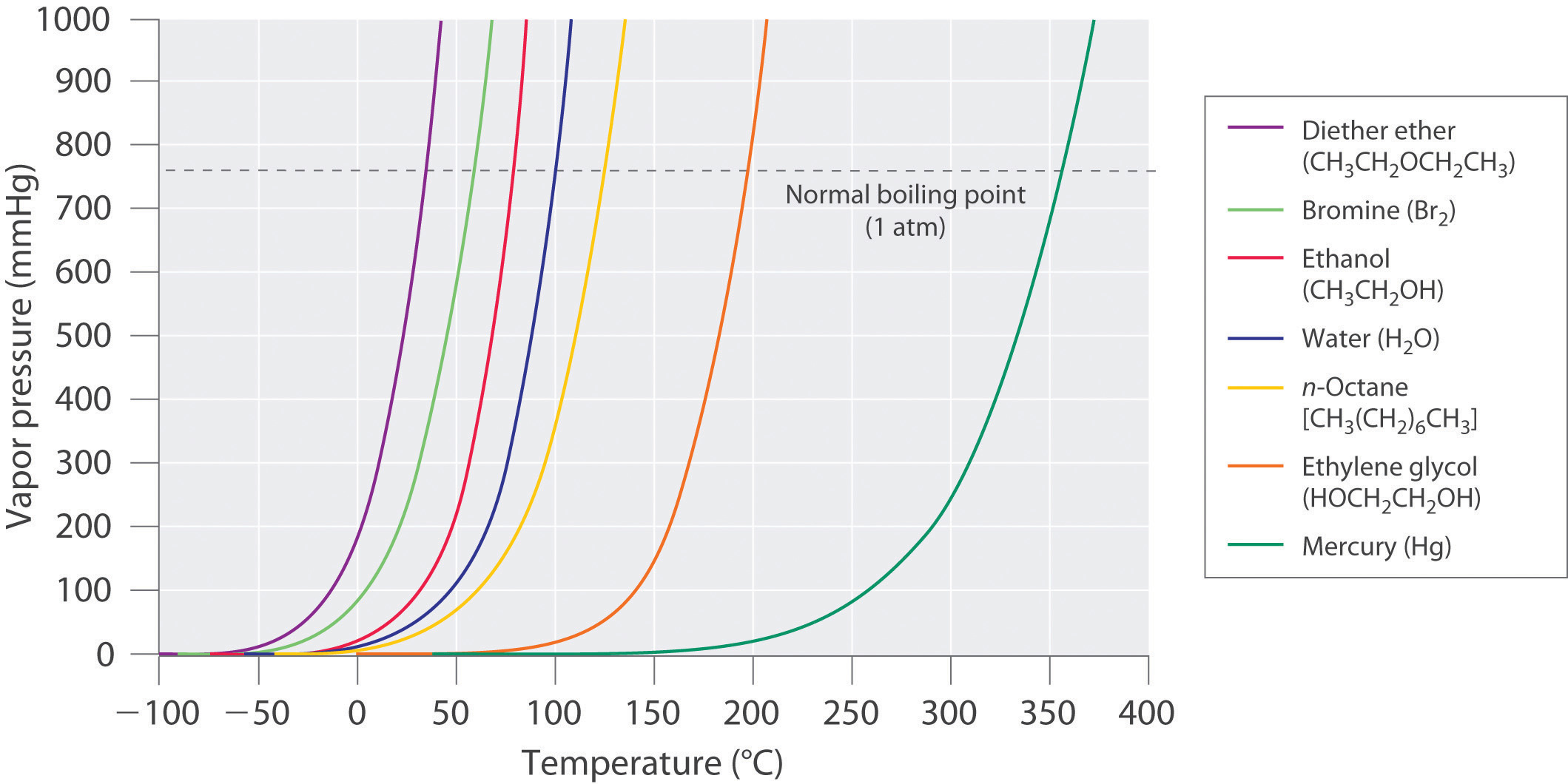
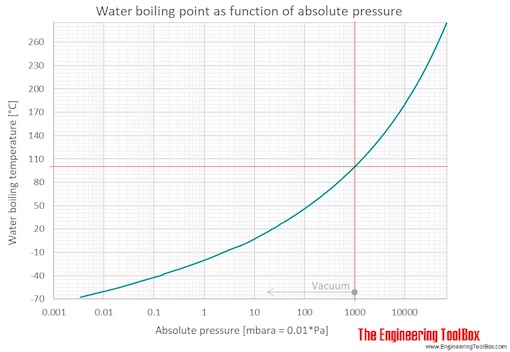
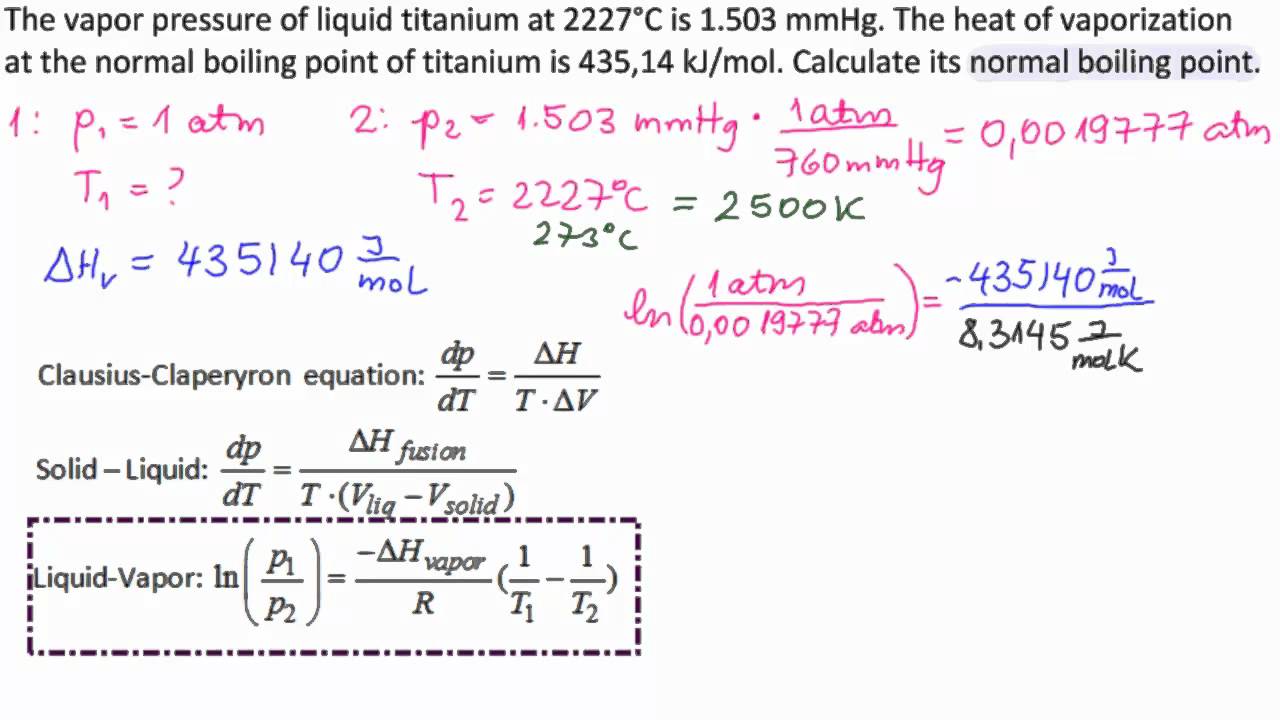
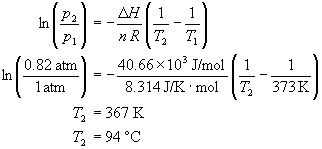Determine the normal boiling point in K of a substance whose vapor pressure is 55.1 mmHg at 23.2^o C and has a ?H_vap of 32.1 kJ/mol. | Homework.Study.com
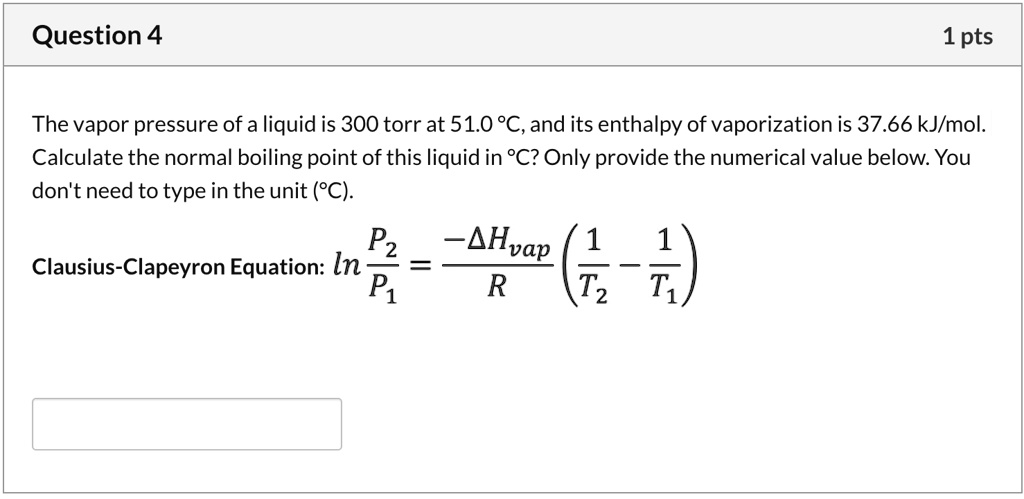
SOLVED: Question 4 1pts The vapor pressure of a liquid is 300 torr at 51.0 *C,and its enthalpy of vaporization is 37.66 kJlmol. Calculate the normal boiling point of this liquid in *